Correct Answer

verified
 _TB4454_00 Resonance structure...
_TB4454_00 Resonance structure...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following is a correct resonance structure for compound A? 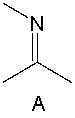
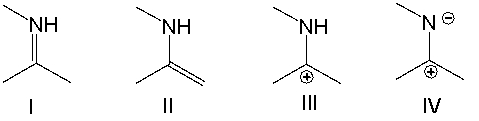
A) I
B) II
C) III
D) IV
E) none of these
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many hydrogen atoms are connected to the indicated carbon atom? 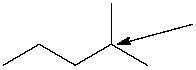
A) one
B) two
C) three
D) four
E) none
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Determine the formal charges on each atom except hydrogen. 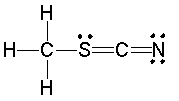
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the relationship between the following compounds? 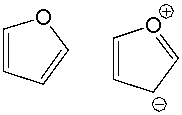
A) constitutional isomers
B) resonance structures
C) conformers
D) identical compounds
E) different compounds
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw a bond-line structure for each constitutional isomer with molecular formula C4H11N.
Correct Answer

verified
 _TB4454_00...
_TB4454_00...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw resonance structures for the following compound. 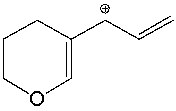
Correct Answer

verified
 _TB4454_00...
_TB4454_00...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following is the correct condensed structure for the following compound? 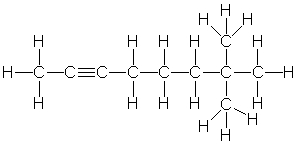
A) CH3C2(CH2) 3C(CH3) 3
B) CH3CC(CH2) 3C(CH3) 2CH3
C) (CH3) 3C2(CH2) 3CH3
D) CH3C≡C(CH2) 3C(CH3) 3
E) CH3CC(CH2) 3C(CH3) 3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds contain an alkyne functional group? 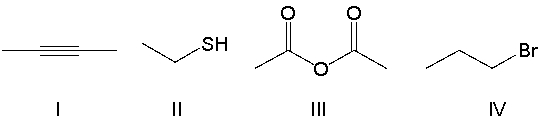
A) I
B) II
C) III
D) IV
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the resonance hybrid of CH2CHCHCHCH2+.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge on the nitrogen atom in the following compound? 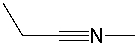
A) +1
B) +2
C) -1
D) -2
E) 0
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the curved arrow(s) for converting the first resonance structure into the second resonance structure. 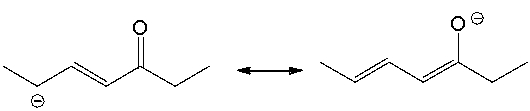
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pairs are resonance structures of each other? 

A) I
B) II
C) III
D) IV
E) None of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct Lewis structure for (CH3) 2CHCH2OH? 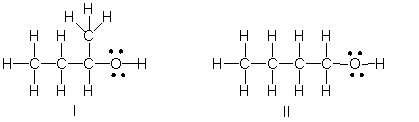
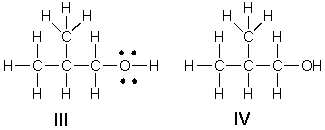
A) I
B) II
C) III
D) IV
E) Both III and IV
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds have +1 as a formal charge on the nitrogen atom? 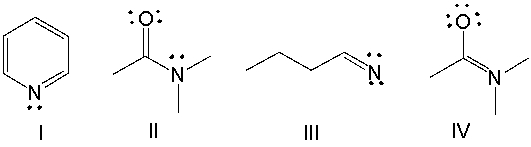
A) I
B) II
C) III
D) IV
E) Both I and II
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds contain an alkyl halide functional group? 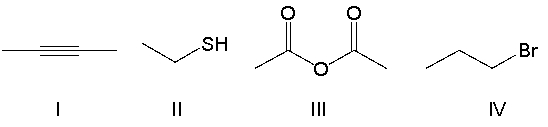
A) I
B) II
C) III
D) IV
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for CH3C≡C(CH2)3C(CH3)3.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the relationship between the following compounds? 
A) constitutional isomers
B) resonance structures
C) conformers
D) identical compounds
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the resonance hybrid of C6H6.
Correct Answer

verified
Correct Answer
verified
Essay
What is a resonance hybrid?
Correct Answer

verified
A molecule that can be represe...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 121 - 140 of 168
Related Exams