A) "1.8 * 105"
B) "385"
C) "1.8 * 10-5"
D) "-1.8* 105"
F) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
____________________ is defined by the following equation. G = -RT ln Keq
Correct Answer

verified
Gibbs free...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following has the highest bond dissociation energy?
A) HF
B) HCl
C) HBr
D) HI
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of the equilibrium constant, Keq, for the following reaction? 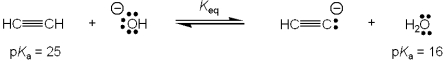
A) "109"
B) "10-9"
C) "9"
D) "1/9"
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the role of ethanol in the acid-base reaction shown? 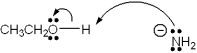
A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the role of ethene in the acid-base reaction shown? 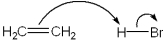
A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species is the conjugate acid in the following acid-base reaction? 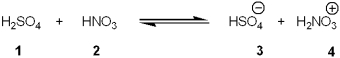
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which set of curved arrows accounts for the deprotonation of an acid (A-H) by a base (:B) ? 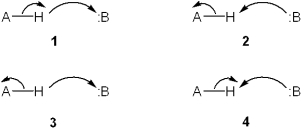
A) 1
B) 2
C) 3
D) 4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery. 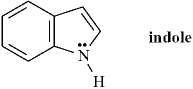 When indole is placed in the presence of a strong base the nitrogen atom will be deprotonated.
When indole is placed in the presence of a strong base the nitrogen atom will be deprotonated.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3NH2
B) CH3PH2
C) CH3OH
D) CH3SH
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the reactivity of boron tribromide, BBr3?
A) Brønsted-Lowry acid and Lewis acid
B) Brønsted-Lowry base and Lewis base
C) Lewis acid and not a Brønsted-Lowry acid
D) Lewis base and not a Brønsted-Lowry base
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium? 
Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
True/False
The following is generic depiction of a reaction using the curve arrow formalism. 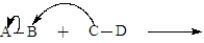 In this reaction electrons move from C to B and A will have a positive charge in the product.
In this reaction electrons move from C to B and A will have a positive charge in the product.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Consider the following reaction coordinate diagram. 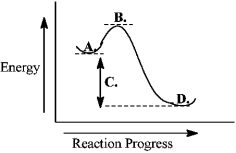 The transition state is represented by the letter B.
The transition state is represented by the letter B.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
The energy needed by reactants to reach the transition state is the____________________,
Correct Answer

verified
free energ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which atom in the following structure is preferentially protonated by a strong acid? 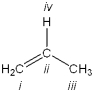
A) i
B) ii
C) iii
D) iv
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Provide the equation for the acid dissociate constant, Ka,for the following equilibrium. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams represents the fastest reaction? 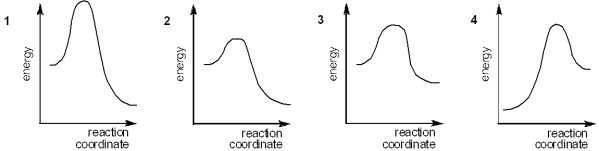
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations is correct?
A) " G = H - T S "
B) " H = G - T S "
C) " G = H - S "
D) " G = H - S /T"
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product. 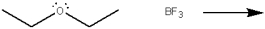
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 94
Related Exams