A) HCl
B) BCl3
C) Br2
D) H2
E) CO2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following constants is/are needed to calculate the amount of energy required to heat 30.5g of H2O(s) at -25.0°C to H2O(l) at 55.0°C? Hfus (H2O) II. Hvap (H2O) III.specific heat of H2O(s) IV.specific heat of H2O(l) V.specific heat of H2O(g)
A) I, II, III, IV, and IV
B) III and IV
C) I, II, III, and IV
D) I, III, and IV
E) I only
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The intermolecular forces present in C6H6 include which of the following? I.dipole-dipole II.ion-dipole III.dispersion IV.hydrogen bonding
A) I, II, III, and IV
B) I and III
C) I, III, and IV
D) I and II
E) III only
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Each of the following substances is a liquid at -50°C.Place these liquids in order of increasing vapor pressure: dimethyl ether (CH3OCH3) , propane (C3H8) , and ethanol (CH3CH2OH) .
A) ethanol < propane < dimethyl ether
B) ethanol < dimethyl ether < propane
C) propane < dimethyl ether < ethanol
D) dimethyl ether < ethanol < propane
E) propane < ethanol < dimethyl ether
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Potassium bromide, KBr, crystallizes like NaCl in a face-centered lattice.The ionic radii of K+ and Br- ions are 133 pm and 195 pm, respectively.Assuming that all Br- ions are positioned in the face and corners of the unit cell, while the K+ ions are positioned along the edge alternating between anions, calculate the length of a unit cell edge.
A) 230 pm
B) 328 pm
C) 523 pm
D) 656 pm
E) 780 pm
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Which would be expected to have the higher boiling point NH3 or PH3?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The boiling points of propanol (CH3CH2CH2OH) and pentanol (CH3CH2CH2CH2CH2OH) are 97°C and 137°C, respectively.The boiling point of butanol (CH3CH2CH2CH2OH) is predicted to be:
A) < 97°C
B) > 137°C
C) > 97°C and < 137°C
D) 97°C
E) 137°C
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Suppose the atoms in a two-dimensional crystal have the following arrangement: 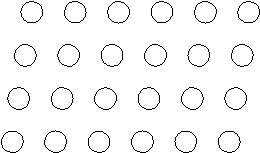 What is the coordination number of each atom in this crystal?
What is the coordination number of each atom in this crystal?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A) PH3
B) He
C) H2S
D) CH4
E) CH3OH
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Which would have the stronger intermolecular forces of attraction H2S or H2Se?
Correct Answer

verified
Correct Answer
verified
Short Answer
Indicate all the types of intermolecular forces of attraction in C2H6(g).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
HOCH2CH2OH(s) is classified as which of the following?
A) metallic crystal.
B) covalent solid.
C) molecular crystal.
D) amorphous solid.
E) ionic crystal.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances is expected to have the highest boiling point?
A) HBr
B) HCl
C) HF
D) HI
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much energy (heat) is required to convert 25.5 g of H2O(l) at 35.0°C to H2O(g) at 115.0°C? 
A) 207 J
B) 7,630 J
C) 8,530 J
D) 9,130 J
E) 65,200 J
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Based on the phase diagram shown below, which is more dense: the liquid phase or the solid phase? 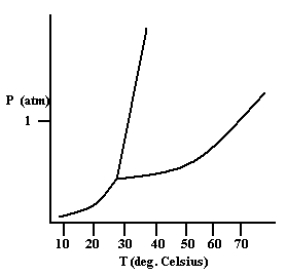
Correct Answer

verified
the solid ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which one of the following substances is expected to have the highest melting point?
A) CH4
B) CCl4
C) CO
D) CO2
E) C(diamond)
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
What phase exists at the point labeled c? 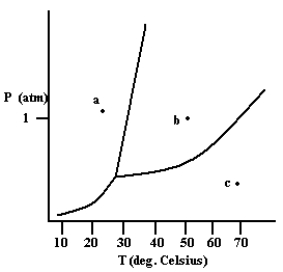
Correct Answer

verified
Correct Answer
verified
Short Answer
Of the given pair of substances at room temperature, which is more viscous? Honey or Mustard
Correct Answer

verified
Correct Answer
verified
Short Answer
Boron nitride, BN3, melts at approximately at 3,000°C under high pressure.This material is almost as hard as diamond.What kind of crystal is this?
Correct Answer

verified
Correct Answer
verified
Short Answer
Indicate all the types of intermolecular forces of attraction in SF4(g).
Correct Answer

verified
dipole-dip...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 101 - 120 of 149
Related Exams