A) The atom has more electrons than protons.
B) The atom has more protons than electrons.
C) The atom has fewer protons than does a neutral atom of the same element.
D) The atom has more neutrons than protons.
E) The net charge is 12.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.
 -Fluorine has an atomic number of 9 and a mass number of 19. How many electrons are needed to complete the valence shell of a fluorine atom?
-Fluorine has an atomic number of 9 and a mass number of 19. How many electrons are needed to complete the valence shell of a fluorine atom?
A) 1
B) 3
C) 5
D) 7
E) 9
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Please refer to Figure 2.1 to answer the following questions.
 -Which drawing depicts an atom that is inert or chemically unreactive?
-Which drawing depicts an atom that is inert or chemically unreactive?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen has an atomic number of 8 and a mass number of 16. Thus, the atomic mass of an oxygen atom is
A) exactly 8 grams.
B) exactly 8 daltons.
C) approximately 16 grams.
D) approximately 16 daltons.
E) 24 amu (atomic mass units) .
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When two atoms are equally electronegative, they will interact to form
A) equal numbers of isotopes.
B) ions.
C) polar covalent bonds.
D) nonpolar covalent bonds.
E) ionic bonds.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Trace elements are those required by an organism in only minute quantities. Which of the following is a trace element that is required by humans and other vertebrates?
A) nitrogen
B) calcium
C) iodine
D) sodium
E) phosphorus
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following questions refer to Figure 2.3.
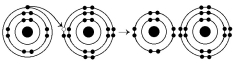 -What results from the chemical reaction illustrated in Figure 2.3?
-What results from the chemical reaction illustrated in Figure 2.3?
A) a cation with a net charge of +1
B) a cation with a net charge of -1
C) an anion with a net charge of +1
D) an anion with a net charge of -1
E) A and D
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.
 -How many neutrons are present in the nucleus of a phosphorus atom?
-How many neutrons are present in the nucleus of a phosphorus atom?
A) 8
B) 15
C) 16
D) 31
E) 46
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the following questions.
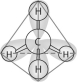 -Which one of the atoms shown would be most likely to form an anion with a charge of -1?
-Which one of the atoms shown would be most likely to form an anion with a charge of -1?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How do isotopes of the same element differ from each other?
A) number of protons
B) number of electrons
C) number of neutrons
D) valence electron distribution
E) amount of radioactivity
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.
 -What is the valence of an atom with six electrons in its outer electron shell?
-What is the valence of an atom with six electrons in its outer electron shell?
A) 1
B) 2
C) 3
D) 4
E) 5
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.
 -How many electrons does an atom of sulfur have in its valence shell?
-How many electrons does an atom of sulfur have in its valence shell?
A) 4
B) 6
C) 8
D) 16
E) 32
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What results from an unequal sharing of electrons between atoms?
A) a nonpolar covalent bond
B) a polar covalent bond
C) an ionic bond
D) a hydrogen bond
E) a hydrophobic interaction
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Please refer to Figure 2.1 to answer the following questions.
 -Atoms whose outer electron shells contain eight electrons tend to
-Atoms whose outer electron shells contain eight electrons tend to
A) form ionic bonds in aqueous solutions.
B) form covalent bonds in aqueous solutions.
C) be stable and chemically nonreactive, or inert.
D) be unstable and chemically very reactive.
E) be isotopes and very radioactive.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the following questions.
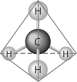 -The molecule shown here is the simplest of organic compounds. It is called
-The molecule shown here is the simplest of organic compounds. It is called
A) a carbohydrate.
B) carbon dioxide.
C) methane.
D) carbonic hydrate.
E) methyl carbonate.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound:
A) HS
B) HS₂
C) H₂S
D) H₃S₂
E) H₄S
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw Lewis structures for each hypothetical molecule shown below, using the correct number of valence electrons for each atom. Determine which molecule makes sense because each atom has a complete valence shell and each bond has the correct number of electrons. Explain what makes the other molecules nonsensical, considering the number of bonds each type of atom can make.
a.
c.
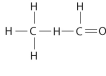 b.
b.
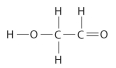 d.
d.
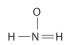
Correct Answer

verified
Correct Answer
verified
Short Answer
Please refer to Figure 2.1 to answer the following questions.
 -Which drawing depicts the electron configuration of oxygen ( O)?
-Which drawing depicts the electron configuration of oxygen ( O)?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be regarded as compounds?
A) H₂
B) H₂O
C) O₂
D) CH₄
E) B and D, but not A and C
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the term trace element, the modifier trace means
A) the element is required in very small amounts.
B) the element can be used as a label to trace atoms through an organism's metabolism.
C) the element is very rare on Earth.
D) the element enhances health but is not essential for the organism's long-term survival.
E) the element passes rapidly through the organism.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 83
Related Exams