A) Ag
B) Al
C) Cu
D) Cd
E) Zn
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
A partial activity series of metals is: Au < Ag < Cu < Pb < Sn. Which ion is most easily reduced: Au3+, Pb2+, Ag+?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is true concerning an oxidation-reduction reaction?
A) The reactant which is reduced is the oxidizing agent.
B) The reactant which is oxidized is the oxidizing reagent.
C) The reactant which gains electrons is the reducing agent.
D) The reactant which loses electrons is the oxidizing agent.
E) None of the statements, A-D, is true.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of the arsenic atom in the AsO43 ion?
A) +1
B) +3
C) +4
D) +5
E) +6
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
When organic compounds that contain sulfur undergo complete combustion with oxygen, one of the products is hydrogen sulfide.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
KMnO4 in acidic media is a powerful oxidizing agent, which is often used in redox titrations.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
A solution was made by taking 2.500 g of KMnO4 and dissolving it in enough water to make 1.000 liter of solution. This solution was used to titrate H2C2O4·2H2O, which can be readily obtained in high purity. In acidic media, the reaction is:2 MnO4-(aq)+ 5 H2C2O4(aq)+ 6 H+ → 10 CO2(g)+ 2 Mn2+(aq)+ 8 H2O How many mL of this solution are required to titrate a 0.480 g sample of H2C2O4·2H2O? Hint: Remember to use moles as an intermediary when performing your stoichiometry calculations for the titration.
Correct Answer

verified
Correct Answer
verified
Short Answer
Three metallic elements, copper, magnesium and gold, can be distinguished from one another on the basis of how they react with two strong acids, HNO3(aq)and HCl(aq). Which set below, using the abbreviations R (for reaction occurs)and NR (no reaction)correctly describes what occurs? 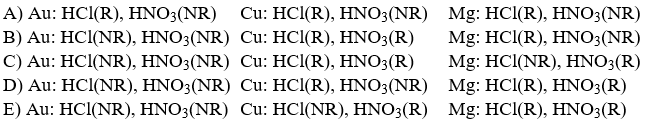
Correct Answer

verified
Correct Answer
verified
True/False
Balancing a redox reaction in acidic media is no different than balancing the same reaction in a basic media.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
A partial activity series is: Au < Ag < Cu < H2 < Pb < Sn < Co < Fe. Which metal will be produced when each of the following compounds reacts with Sn metal:Co(NO3)2(aq); Fe(NO3)2(aq); HNO3(aq); Cu(NO3)2(aq); Sn(NO3)2(aq)?
Correct Answer

verified
Correct Answer
verified
Short Answer
When a metal is reacted with nitric acid, which element in nitric acid is reduced?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element would react most rapidly with water?
A) K
B) Cd
C) Cs
D) Na
E) Zn
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Which substance in the reaction 5 H2O(l)+ 4 Cl2(g)+ S2O32-(aq)→ 2 SO42-(aq)+ 8 Cl-(aq)+ 10 H+(aq)is the reducing agent?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When aluminum is exposed to the environment it does not corrode in the same way as iron. Which of the following best explains why? Hint: Look at the chemical properties of iron and aluminum.
A) Aluminum quickly oxidizes at the surface and forms a protective coating that prevents further oxidation.
B) Aluminum is not as reactive as iron, and does not react with molecular oxygen.
C) Aluminum is not easily reduced, so it is not reduced by exposure to oxygen.
D) Aluminum normally treated with a polymer coating that prevents oxidation.
E) Aluminum reacts with water to form a hydride coating that protects the rest of the aluminum from oxidation.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Which reaction below does not occur spontaneously upon mixing the reagents shown?
A) Cd(s) + Al3+(aq) Cd2+(aq) + Al(s)
B) Cd(s) + Cu2+(aq) Cd2+(aq) + Cu(s)
C) Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s)
D) Al(s) + Ag+(aq) Al3+(aq) + Ag(s)
E) Cu(s) + Au3+(aq) Cu2+(aq) + Au(s)
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Which reaction below occurs spontaneously upon mixing the reagents shown?
A) Sn(s) + Zn2+(aq) Sn2+(aq) + Zn(s)
B) Ag(s) + Mg2+(aq) Ag+(aq) + Mg(s)
C) Zn(s) + Au3+(aq) Zn2+(aq) + Au(s)
D) Ag(s) + Zn2+(aq) Ag+(aq) + Zn(s)
E) Sn(s) + Al3+(aq) Sn2+(aq) + Al(s)
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Based on this list, which element below would undergo oxidation least readily?
A) Mg
B) Al
C) Cu
D) Cd
E) Zn
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Identify the reducing agent in the chemical reaction Cd + NiO2 + 2H2O → Cd(OH)2 + Ni(OH)2.
Correct Answer

verified
Correct Answer
verified
Short Answer
What two products are formed when CH4 reacts with a very limited supply of molecular oxygen? Hint: Oxygen must be conserved in the products.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You can dissolve a penny using nitric acid, HNO3. The reaction Cu(s) + 4 HNO3(aq) Cu(NO3) 2(aq) + 2 NO2(g) + 2 H2O(l) is used to dissolve copper metal. If there is 1.000 g of copper to be dissolved, and instructions say to use four times as much acid as the required amount, how many mL (to the nearest mL) of 15.0 M nitric acid would be required to dissolve the copper? Hint: Don't forget the coefficients in the balanced reaction above when performing your calculations with nitric acid.
A) 4 mL
B) 5 mL
C) 10 mL
D) 17 mL
E) 25 mL
G) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 175
Related Exams